Takeaways
- The information and farming practices established for dairy cattle are not directly applicable to dairy goats.
- Characteristics of high-quality milk in dairy goats are low bacteria counts, acceptable somatic cell count numbers, and ideal flavor and appearance.
- A good milk harvest and storage protocol to reduce bacteria consists of four major components: clean udders, clean hands, clean equipment, and milk temperature.
- The taste, smell, and texture of milk are highly influenced by milk components such as protein, lactose, and fat.
Producing high-quality milk in dairy goats requires species-specific insight. Because of nuances in physiology and management, the information and farming practices established for dairy cattle are not directly applicable.
Although production systems may vary from producer to producer, the core characteristics of high-quality milk in dairy goats include: 1) milk with low bacteria counts, 2) milk with acceptable somatic cell count numbers, and 3) milk with ideal flavor and appearance. While these factors can be interrelated, this article will assess them individually, with an emphasis on strategies for milk quality evaluation and improvement.
Achieving high milk quality is not only essential for the health of the animal and optimal production; it is also critical for adhering to regulatory parameters and maintaining customer satisfaction.
Maintaining Low Bacteria Counts in Milk
Reducing bacteria in milk is important as it often influences its flavor and shelf life. To monitor bacteria levels in milk, farmers can observe changes in flavor, smell, or stability as early indicators of potential issues. If a problem is suspected, samples can be sent to a certified lab for accurate bacterial count analysis.
If milk is moving off the farm for interstate commercial sales of Grade A milk, bacterial counts must meet standards outlined in the Pasteurized Milk Ordinance (PMO; U.S. Food and Drug Administration, 2023). If milk is remaining within the state (intrastate), milk quality guidelines are provided by the state’s department of agriculture or an equal governing body.
Given the natural sugar content of milk, bacteria can grow rapidly; therefore, it is imperative to quickly pinpoint and eliminate possible causes of bacterial contamination. High bacteria counts in milk generally originate from two primary sources: infections within the udder, such as mastitis, and external contamination during the milking or storage process. While mastitis is covered later in the publication, this section focuses on the relationship between milking hygiene and bacterial levels.
A good milk harvest and storage protocol to reduce bacteria consists of four major components:
Clean Udders
Keep hair on the udder and flank clipped, as this will improve the overall cleanliness of the milk harvesting process. Just prior to milking, the udder should be cleaned by removing any dirt and debris. The udder should also be dry, as bacteria are more likely to spread in a moist environment.
The next step involves stripping foremilk or removing the first three to five streams of milk from each gland. Stripping foremilk allows for 1) initiation of milk letdown if not already initiated, 2) the milker to check for abnormalities in the milk, which may signify mastitis, and 3) the removal of milk with the highest bacterial and somatic cell counts.
Next, a pre-dip should be applied to reduce the environmental bacteria load on teat ends and help reduce the transfer of contagious pathogens between animals during milking. Iodine-based dips are the most prevalent, but other options include chlorhexidine or hydrogen peroxide. With all teat dips, the dip should cover a minimum of two-thirds of the teat and have a minimum contact time of 30 seconds. Teat dips should be thoroughly removed before milking with either a disposable towel or a washed towel.
Following milking, the application of post-dip will reduce the potential for bacterial entry into the udder while the teat sphincter is relaxed, for approximately 30–60 min after milking. Post-dips often have the same base disinfectant as pre-dips but contain it in higher concentrations. Post-dip will also have surfactants to aid in surface contact, and teat conditioners. Post-dip should be applied in the same manner as pre-dip; however, post-dip is not wiped off or removed, but left on the teat.
Clean Hands
Hands can introduce bacteria to the udder and spread bacteria between udders if milking multiple animals. Wearing gloves during milking is highly recommended. Gloves can reduce the introduction and spread of bacteria by eliminating locations for bacteria to travel (e.g., cracks of hands, under fingernails). The smooth surface of gloves makes them easier to disinfect between goats using either disinfectants or teat dips. If choosing not to wear gloves, hands should be cleaned with a disinfectant between animals.
Clean Equipment
The sugar content in milk makes it an excellent food source for bacteria, and many bacteria can survive across a wide range of temperatures. Because of these factors, all milk residues should be thoroughly removed from milking equipment immediately after use. Most automatic cleaning systems include a pre-rinse, alkaline (detergent) wash, acid wash, and a sanitizing rinse to remove milk residue and remove or kill any bacteria. If hand cleaning, these same steps should be followed (Table 1).
Table 1. Steps to Clean Milking Equipment.
| Cleaning Step | Specifics | Purpose |
|---|---|---|
| Prerinse | Warm water rinse. The ideal temperature is considered lukewarm or 100–110 °F. Thoroughly rinse immediately after milking is finished. | Removes any remaining milk or debris in the lines without creating any films on the surface. Films from proteins form when water is too hot, while films from fat form if water temperature is too low. |
| Alkaline (detergent) wash | Mix detergents according to the manufacturer’s directions. Wash water should be hot. In commercial systems this would be 170 °F. The minimum cleaning time is 6–10 min. If not using forced movement of wash water through lines and equipment, you must disassemble all pieces of equipment to wash. Another option is to make a bucket of wash water and turn on the milking unit to move the wash water through. Be mindful of the wash water temperature, as a minimum temperature of 120 °F is necessary to prevent adherence. | Removes most biofilms and buildups from residual milk components such as fat and protein. Biofilms are often referred to as microorganisms (mostly bacteria) that adhere to surfaces, creating a slimy, glue-like matrix. |
| Acid rinse | A minimum contact time of 2–3 min is required with the acid rinse being a pH of 3.0–4.0. | Removes all remaining wash solution, prevents milk minerals (milk stone) from depositing on surfaces, and serves as a biostatic to inhibit bacterial growth. |
| Dry | Equipment should be placed in a clean location to dry. This might require hanging the equipment, which allows all water to move through and out of tubing. | Drying limits bacterial growth between milkings. |
| Sanitizing | Most sanitizing solutions are chlorine-based. Water temperature should be 100–110 °F. | This step is performed prior to the next milking and ensures removal of any lingering bacteria. |
Milk Temperature
Bacteria thrive in milk not just because of its sugar content but also because of its warm temperature. To reduce bacterial load, the PMO mandates that milk temperature be reduced to or below 50 °F within 4 hr of initiating the milking process. Within an additional 2 hr, milk must be at or below 45 °F. Temperature requirements for the maintenance of milk as a raw product for intrastate use, if legal, may vary based on the regulatory documents of individual states.
Maintaining Acceptable Somatic Cells in Milk
Somatic cells in milk are primarily white blood cells and represent part of the body’s natural defense against infection. However, somatic cells may also include other cell types, such as epithelial cells from the lining of the udder. Therefore, somatic cells are inherently present in milk as part of milk production and the animal’s immune response, but elevated levels can indicate an issue with udder health.
Goats are known for naturally higher somatic cell counts (SCC) relative to their dairy cow counterparts, as they shed more cells as part of the milk production process and experience greater fluctuations relative to their environment, feed, reproductive status, and stage of lactation. Therefore, standard metrics used to assess SCC in the dairy cattle industry are not directly applicable to the dairy goat industry. The PMO reflects this difference, and allows dairy goat milk a legal SCC threshold at 1,500,000 cells/ml, as opposed to the 750,000 cells/ml allowed in dairy cow milk.
When SCC rises above what is biologically normal, it typically indicates mammary inflammation, more commonly known as mastitis. While mastitis may result from trauma to the udder, it is more commonly caused by an infection. For this discussion, the focus will be on infectious causes of mastitis.
The most common infectious causes of mastitis in dairy goats are pathogenic species of Streptococcus, Staphylococcus, and coliforms (Tibebu et al., 2025). While characteristics of the infection (presentation, duration) may give some insight into the potential pathogen source, it can only be conclusively determined by laboratory culture and analysis.
There are numerous preventative methods for mastitis. While no method is fail-safe, adherence to those listed below will reduce the likelihood of infection.
Clean Living Environment
The living environment should be clean of manure, mud, and excess moisture. Signs that the environment needs cleaning include the smell of ammonia, bedding adhering to the lower limbs or body of the animal, moisture on clothing when kneeling in bedding, and/or a dirty udder at milking.
- If housed in a barn or under cover, topdressing clean bedding material as needed with complete bedding removal and replacement several times a year is generally recommended. If using outdoor housing, does should have access to well-drained and clean areas.
- Organic materials (e.g., shavings or straw) are a prime site where mastitis-causing pathogens may germinate and live outside of the body. Therefore, organic materials need to be either properly composted as bedding or regularly cleaned. Sand is an example of an inorganic bedding option.
- Unclean living environments are attractants to flies, which are known to transmit mastitis-causing pathogens.
- Enclosed housing requires adequate ventilation and humidity control, as high moisture areas foster bacterial growth.
Clean Milking Procedure
The milking procedure should protect both the mammary gland and the milk produced from bacterial contamination.
- Wear gloves when milking.
- Properly clean milking equipment.
- Use both pre- and post-dips. While the pre-dip is intended to clean the teat before milking, the post-dip serves to moisturize and protect the mammary gland from bacterial colony formation after milking. Because the teat sphincter can remain open for up to an hour after milking, the protective barrier provided by the post-dip helps safeguard the gland during this vulnerable period.
Milking Machine Maintenance
Milking-machine maintenance is critical to maintaining a healthy mammary gland.
- Check the inflations (liners) of the milking claw regularly for any cracks, damage, or twisting. Damaged or worn inflations can slow the milking process, result in incomplete milkings, harbor bacteria within cracks or holes, and damage the teat ends, all of which can cause mammary health issues. The life of inflations varies based on their material, frequency of use, and how well they are maintained. Most inflations come with manufacturer’s guidelines on how many milkings they are warranted for. Even so, routine checking of inflations is recommended for optimum performance and mammary health.
- It is critical to maintain the correct vacuum pressure and pulsation rate during milking to minimize damage to the teat end and ensure the inflations stay on the teat. When the latter fails, it is often called a liner slip. During a liner slip, air can enter the inflations. Anything in the air then has access to the interior of the teat and, consequently, the mammary gland. Recommended vacuum pressure generally falls between 36–48 kPa, with a pulsation rate of 70–120 cycles per minute and a pulsator ratio of 60% (Romero et al., 2021). Variations in pressure and pulsation rate and ratio will vary by machine and farm. To determine optimum pressure, rate, and ratio, producers should assess the frequency and amount of residual milk and the frequency of liner slips, as well as evaluate teat-end health and color following each milking and over time.
Reduce Fly Populations
Flies are vectors for mastitis-causing pathogens, and all efforts should be taken to reduce their populations in the living, feeding, and milking environments of goats.
- Feed is one of the primary attractants of flies. Be sure to remove leftover feed and dispose of it in a location that is not close to your animal housing or milking locations.
- Forced air movement, such as with fans, reduces fly populations in barns and covered housing facilities.
- Insecticides approved for lactating does may be used in various forms (e.g., spray, dust) to reduce fly populations on animals.
- Fly baits or predators may also be considered but have variable results based on several factors, including the environment, housing system, and the number of animals.
Animal Cleanliness and Health
Keep nursing animals clean and healthy, as pathogens may be spread by offspring. If using a doe to milk and allowing offspring to nurse, be aware that pathogens may orally transfer to the doe. Some of those pathogens may be inherently harmful to the offspring (Pasteurella hemolytica), while others may just cause an infection in the doe.
Reduce Stress and Other Illnesses
When a doe is stressed or battling other health challenges, infections may have a greater chance to establish and replicate rapidly within the mammary gland. The general term for this condition is immunocompromised, and it results in lowered immune defenses to fight pathogens.
Isolate Infected Animals
As a component of biosecurity, does with known infections should be kept out of the main milking herd and milked last. This reduces the potential spread of infections to uninfected does. If necessary, the animal may also be milked into a separate vat to protect the main milk line from elevated bacteria and SCC.
Identify and Treat Quickly
Rapid identification and treatment of mastitis issues will reduce their impact on milk quality and quantity. Clinical cases of mastitis are most often identified by:
- Abnormal consistency of milk (watery or thick), potentially including flakes and clots, and/or a change in milk color.
- A hot or swollen gland. In the case of gangrenous mastitis, the gland may become cold.
- If the infection is systemic, does will likely show other signs of illness, including lethargy, loss of appetite, and fever.
Subclinical cases are often the hardest to identify, as they go unrecognized without any noticeable physical changes to the milk or doe. The most common sign of a subclinical infection is reduced milk production.
Mastitis may be in one half of the udder and not the other. If this is the case, practicing good milking hygiene may prevent spread from the infected half to the uninfected part. Halves should be evaluated and treated individually.

A useful and cost-effective tool for mastitis detection is the California Mastitis Test (CMT; Figure 1), which uses a reagent to disrupt the membrane and bind the DNA of somatic cells. The thicker the reagent becomes, the higher the SCC. As explained in Table 2, a gelatinous, thick outcome often means a case of mastitis.
It is important to recognize that standard interpretation for dairy cattle cannot be used in dairy goats, remembering that goats naturally have a much higher SCC in milk. Consult with your veterinarian regarding treatment options.
Table 2. Interpretation of California Mastitis Test Scores in Goats.
| CMT score | CMT Interpretation | Visual characteristics of the milk | SCC Range (cells/ml) | Suggested interpretation |
|---|---|---|---|---|
| N | Negative | There is no change to the mixture. It remains liquid in consistency with color similar to when the reagent solution was first added. Pours out as liquid. | 0–200,000 | Normal, healthy gland. Retest monthly. |
| T | Trace | Mixture thickens slightly and moves around the paddle as liquid when swirled. Pours out as liquid. | 150,000–500,000 | Likely a normal, healthy gland but may need to retest if any symptoms of mastitis present and/or milk production declines. Otherwise, retest monthly. |
| 1 | Weak | Mixture thickens slightly but does not form a gel. Movement around the paddle changes slightly, with some clinging to the side walls of the wells. Pours out at a steady pace. | 400,000–1,500,000 | Growing concern regarding an infection but could be normal for the doe. Retest weekly. If score progresses to 2 or 3, consult with a veterinarian. |
| 2 | Distinct | Gel is forming and when the mixture is swirled in the well, there is a tight clump that remains in the center. Pours out with heavy gel first but some liquid may remain in the well. | 800,000–5,000,000 | High likelihood of infection. Retest in 3 days. If high again, consult with a veterinarian. |
| 3 | Strong | Mixture is completely gelled and clumps in the middle when the mixture is swirled. Pours out as one cohesive gel mass with no liquid remaining in the well. | > 5,000,000 | Infection. Consult with a veterinarian. |
| Note. Some changes in scores might be noted based on normal physiological changes. The monthly testing of does will help delineate what is normal for the doe and/or herd versus what is abnormal. | ||||
Antibiotics
Consider a selective intramammary antibiotic therapy at dry-off. This therapy uses antibiotics at the time of dry-off only in does or udder halves with known mastitis issues. The use of antibiotics will help treat the existing infection while aiding in the prevention of new infections. Selective therapy at dry-off should be conducted in consultation with a veterinarian for antibiotic selection and withdrawal periods.
Achieving Milk with Ideal Flavor and Appearance
The taste, smell, and texture of milk are highly influenced by milk components such as protein, lactose, and fat, which in turn are affected by the presence (or absence) of mastitis, goats’ diets, and their genetics.
The Role of Mastitis
Milk flavor and appearance is a representation of its components, primarily protein, lactose, and fat. As previously discussed, reducing mastitic infections is important for animal health, milk cleanliness, and milk stability. Producers should also understand the role that the inflammatory process can have on milk components, which ultimately alter many of milk’s sensory characteristics. In short, the defensive actions of the mammary gland are costly to milk components.
Mastitic infections markedly increase proteolytic blood enzymes. Of primary interest is the increase in the enzyme plasmin, an activated form of plasminogen. Increased levels of plasmin in milk are associated with decreased milk production and increased casein degradation. Casein is the major protein found in milk and contributes to its white color and creaminess. Outside of these sensory characteristics, a reduction in casein subsequently causes a reduction in cheese yield, as it plays a vital role in milk coagulation and curd formation.
Mastitic infections also decrease the lactose content in milk. Mastitis damages the epithelial cells lining milk-secreting cells. This disruption can result in reduced movement of glucose and galactose (the two sugars that make up lactose) into these cells. Additionally, animals with mastitic infections may have reduced feed consumption, leading to less available glucose. Both factors can reduce the lactose content of milk alone or in combination. Lactose is important for the sweetness and flavor of milk. Additionally, lactose is the main osmotic component drawing water into the mammary gland. Thus, a reduction in lactose leads to a reduction in water and total milk production. This is why lactose is often referred to as the “milk valve.”
Finally, fat is important for the taste and consistency of milk. Oxidative stress, a part of the inflammatory reaction, impacts the mammary gland and its products. Oxidative stress increases peroxidation, which is the breakdown of fats and not only decreases their content but also may impact the milk’s flavor profile, causing off flavors and reducing the yield of products made from milk. The reduction amount may vary based on the stage of lactation for the animal, realizing that components such as fat become more concentrated at the end of lactation.
The Role of Forages
The conversation regarding nutrition programs for goats is diverse and robust given breed differences, available forage, and management styles. This discussion will focus on the influence of certain feeding strategies that will influence the sensory characteristics of goat milk.
Milk, in its quantity and components, is a direct reflection of the feed quality and quantity provided to the animal. Any nutritional inadequacies are reflected in lowered production, changes in milk composition, and reduced product yield (i.e., cheese, yogurt). Therefore, it is essential to provide a nutritional program that meets nutritional needs for growth (if a young animal), maintenance, and lactation.
Many goats raised for milk production are housed on pasture. While pasture-rearing goats is an acceptable practice, it is important to understand the influence of pasture systems on milk production and composition, the latter of which can affect the flavor and appearance of milk.
The impact of compositional differences was previously discussed in the section on mastitis. First, the quality and quantity of forage may influence milk. With seasonal variations in forage availability, it is important to supplement animals on pasture when requirements are not met with forage alone. This supplementation may be high-quality stored forage, a byproduct feed, or a grain mix. An ideal way to assess this need is to determine total forage availability and quality. Please see “Related Expert Resources” for suggested reading on forage availability and quality. In diverse forage systems, it may be difficult to characterize nutrient availability and how it changes over time.
When thinking of a forage plan, also recognize that there are changing nutrient requirements for does based on stage of lactation and pregnancy (Figure 2).
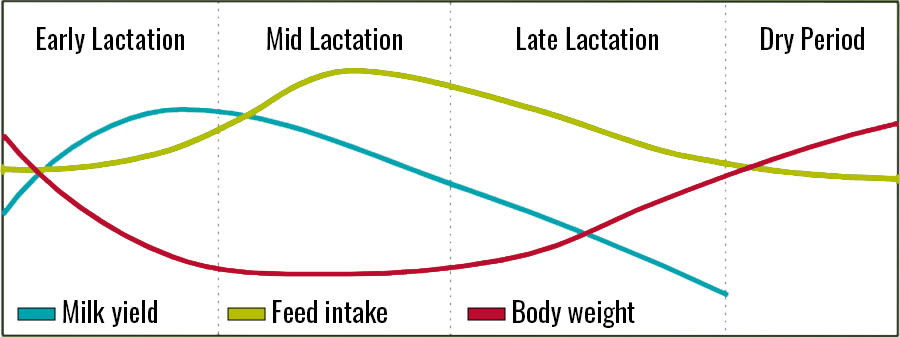
The highest nutrient requirement for lactating does is in the first 6–8 weeks of lactation. During this time, most does will prioritize both energy and nutrients for milk production. In most cases, even nutritionally dense diets cannot prevent does from losing body condition. Around 10–12 weeks of lactation, does will begin to increase in feed intake while also leveling off or decreasing in milk production. The result is typically a more consistent body condition. Toward the end of lactation, as milk production continues to decline, body condition will conversely increase.
Breed, health, lactation length, and supplementation strategies may alter this pattern to varying degrees. High-energy and protein diets are required for high milk production. However, fiber is required not only for rumen health but also for increased fat content in milk. Please see “Related Expert Resources” for suggested reading on goat nutrition for milk production.
Something else to consider with goats is that the flavor profile of milk may change based on available forages. The free fatty acid profile of milk changes based on available feeds. Goat milk contains higher levels of medium-chain fatty acids than cow milk does, which allows their milk to be more capable of assuming various aromas and taste. These profiles then alter the milk flavor. While that alteration in milk flavor is advantageous for making certain cheeses, it can be a hurdle when trying to achieve specificity or consistency in flavor profiles. Milk flavor may also be changed by aromatic feed such as onions, fool’s parsley, brassicas, etc. Additionally, milk is known to absorb odors; thus, many report a musky flavor of milk when bucks are housed near does or the milking location, especially during breeding season.
The Role of Breeds and Genetics
Just like genetics and breed-specific traits influence milk production in dairy goats, they can also influence factors that affect milk taste and quality, like milk component composition, SCC tendencies, disease resistance trends, and the overall flavor and texture of milk and cheese.
While each producer must evaluate their production capabilities and tailor their operation to a desired product or goal, certain dairy goat breeds do present specific advantages.
- Nubian goats are famously heat-tolerant but are specifically known to produce the highest butterfat percentage on average (for standard-sized goats). Their milk is valued for its creaminess and generally used to make cheese.
- Alpine goats are specifically known for their year-round milk production capabilities and for possessing a clean flavor that is free from strong aromas.
- The high protein content, calcium-enhanced milk of LaMancha goats makes it easier to digest and ideal for high cheese yields.
- Saanen goats are known for their high volume production with lower fat percentages, making them ideal for commercial milk operations.
- Nigerian Dwarf goats are the top butterfat producers, producing a low quantity but high-fat-component milk.
The Role of Postharvest Milk Handling
Poor handling of raw milk after harvest can greatly influence the flavor, smell, and appearance of milk. It is vital that raw milk is stored in a clean, metal container (preferably a bulk tank) to prevent bacterial growth. The milk must be rapidly cooled and should undergo regular microbial testing to ensure its cleanliness and freshness.
Please refer to the previous section on reducing bacteria counts for methods to properly handle milk during the harvest and postharvest period.
Final Tips and Considerations
- Keep animals, their environment, and the milking area clean.
- Regularly check teat-end health. Roughened teat ends may indicate an issue with milking machine vacuum pressure, which can increase somatic cell counts and the potential for mastitis.
- Use fresh does to dilute milk. This may mean you milk does at various stages of lactation to dilute components that may become highly concentrated at the end of lactation, especially if in an extended lactation. This might also help dilute any issues in SCC with an individual doe.
- Check for residual milk. Residual milk is milk remaining in the udder after milking and is problematic for two reasons: It will naturally start to dry the doe off, and it will serve as a remaining food source for bacteria. Does require different milking times, as they milk at different speeds and with different volumes. Therefore, always palpate the udder to determine when to shut off the vacuum (or cease hand milking) and routinely check for residual milk.
- Do not panic if SCC rises. Some fluctuations might be natural because of environmental or management changes. Other fluctuations may involve an infection that will naturally clear. Milk culturing will indicate if the rise is the result of an infection, and if so, help identify the pathogen causing the infection, which will allow for a more specific treatment plan.
- Keep consistent and thorough records of cleaning and milking protocols, individual mastitis cases, general SCC trends, CMT results, and any treatment or antibiotic usage. This will prevent mistakes in management practices and can help track animal and herd health by indicating a doe with chronic mastitic infections or illuminating a growing contagious pathogen problem.
Conclusion
Producing high-quality milk in dairy goats requires attention to detail, consistency in management, and a clear understanding of the unique needs of the species. By prioritizing clean and efficient milking practices, maintaining animal health, and supporting production through proper nutrition and environmental management, producers can significantly enhance milk quality.
While some variability is inevitable, especially in small ruminants, producers should focus on three key areas. These are bacterial control, maintaining acceptable somatic cell counts, and optimizing flavor and appearance. For more information, contact your local Extension office.
Related Expert Resources
Bradley, B. (2019, August 12). Dairy goats and sheep: Goat and sheep nutrition and forages. Alabama Cooperative Extension System. https://www.aces.edu/blog/topics/sheep-goats/dairy-goat-and-sheep-nutrition-and-forages/
Hancock, D., & Andrae, J. G. (2017). Grazing impacts on pasture composition. (Publication No. B 1243). University of Georgia Cooperative Extension. https://fieldreport.caes.uga.edu/publications/B1243/
Hancock, D., Saha, U., Stewart, R. L., Bernard, J. K., Smith, R. C., Johnson, J. M. (2017). Understanding and improving forage quality (Publication No. B 1425). University of Georgia Cooperative Extension. https://fieldreport.caes.uga.edu/publications/B1425/
References
Annis, F. M., & McDougall, S. (2002). Efficacy of antibiotic treatment at drying off in curing existing infections and preventing new infections in dairy goats. Proceedings of the New Zealand Society of Animal Production, 62, 19–21. https://www.nzsap.org/system/files/proceedings/2002/ab02006.pdf
Castro, N., Suarez-Trujillo, A., Gonzalez-Cabrera, M., Hernandez-Castellano, L., E., & Argüello, A. (2023). Goat lactation research as a gateway for the development of the dairy goat industry. Animal Frontiers, 13(3), 108–111. https://doi.org/10.1093/af/vfad005
Desidera, F., Skeie, S. B., Devold, T. G., Inglingstad, R. A., & Porcellato, D. (2025). Fluctuations in somatic cell count and their impact on individual goat milk quality throughout lactation. Journal of Dairy Science, 108(1), 152–163. https://doi.org/10.3168/jds.2024-25310
Menzies, P. (2021). Udder health for dairy goats. Veterinary Clinics of North America: Food Animal Practice, 37(1), 149–174. https://doi.org/10.1016/j.cvfa.2020.12.002
Merkel, R. C., Gibson, T. A., & Sahlu, T. (Eds.). (2016). Dairy goat production handbook. Langston University.
Novac, C. S., & Andrei, S. (2020). The impact of mastitis on the biochemical parameters, oxidative and nitrosative stress markers in goat’s milk: A review. Pathogens, 9(110), 882. https://doi.org/10.3390/pathogens9110882
Romero, G., Bueso-Ródenas, Alejandro, M., Moya, F., & Díaz, J. R. (2021). Effect of vacuum level and pulsation parameters on milking efficiency and animal welfare of murciano-granadina goats milked in mid-line and low-line milking machines. Animals, 12(1), 40. https://doi.org/10.3390/ani12010040
Seges Innovation. (2019, November 14). Bacteria in the milk – and what to do about an increased total bacteria count. Landbrugsinfo. https://www.landbrugsinfo.dk/public/e/8/f/malkning_malkekvalitet_bacteria_in_the_milk
Tibebu, A., Teshome, Y., Tamrat, H., & Bahiru, A. (2025). Mastitis in goat: A review of etiology, epidemiology, economic impact, and public health concerns. One Health, 21, 101131. https://doi.org/10.1016/j.onehlt.2025.101131
U.S. Food and Drug Administration. (2023). Grade “A” pasteurized milk ordinance (PMO). Department of Health and Human Services. https://www.fda.gov/media/180975/download?attachment
Yakan, A., Özkan, H, Baran, Ç., Kaya, U., Karaaslan, İ., & Dalkiran, S. (2021). Expression patterns of major genes in fatty acid synthesis, inflammation, oxidative stress pathways from colostrum to milk in Damascus goats. Scientific Reports, 11(1), 9448. https://doi.org/10.1038/s41598-021-88976-0









