Poultry processors incorporate numerous antimicrobial interventions in the slaughter process to reduce the prevalence and concentration of the foodborne pathogens Salmonella and Campylobacter. In some cases, the goal is to meet regulatory performance standards for Salmonella and Campylobacter enforced after the chilling step for whole carcasses, and before and after packaging poultry parts. The conventional process is to evaluate the efficacy of the incorporated antimicrobial interventions in reducing either indicator microorganisms or the foodborne pathogens immediately after the intervention step.
Poultry processors are faced with decisions about which antimicrobial to use for effective control of Salmonella and Campylobacter on whole birds and chicken parts. There are several antimicrobials (i.e., “chemistries”) available, however, choosing one that works well with existing processes can define a successful pathogen control program.
Validation is the process of demonstrating that the design of a hazard analysis and critical control point (HACCP) system can adequately control identified hazards to produce a safe, unadulterated product.
Distinct elements of validation
- Supporting scientific information: scientific or technical support for the HACCP system
- Practical demonstration: the initial, practical in-plant demonstration proving that the HACCP system can perform as expected
Some of the common controls that should be validated in a poultry processing plant are critical control points (CCPs) identified in the HACCP plan, prerequisite program interventions (good manufacturing practices, or GMPs; standard operating procedures, or SOPs; and sanitation standard operating procedures, or SSOPs) preventing a hazard from being likely to occur, purchase specifications, and product formulations (for further processing facilities) where the formulation contributes to the safety of the product.
Supporting scientific information
Its use for process validation is an easy and effective starting point for the validation process. Scientific information or technical support for a control system should be complete, reviewed by regulatory agencies, and can consist of, but is not limited to, peer-reviewed journal articles, a documented study, in-house data, or data generated from published guidelines.
Practical demonstration
In-plant observations, measurements, microbiological test results, or other information demonstrating that control measures, as written into a HACCP system, can be implemented to achieve the intended results. One example is meeting Salmonella and Campylobacter performance standards for whole carcasses and parts. The U.S. Department of Agriculture Food Safety and Inspection Service (USDA-FSIS) states that validation data for any HACCP system must include practical data or information reflecting an establishment’s actual experience in implementing the HACCP system. In-plant validations include gathering data to demonstrate that the collection of interventions and process steps together in sequence produce a safe, wholesome unadulterated product. For example, is the HACCP system achieving the desired result? Processing plants should collect data related to the food safety hazards identified in the HACCP plan when a new HACCP system is implemented, or whenever a new or modified food safety hazard control is introduced into an existing HACCP system.
The goal should be to gather necessary data by repeatedly testing adequacy of the process and to
establish that the HACCP system meets the designed parameters to achieve intended results.
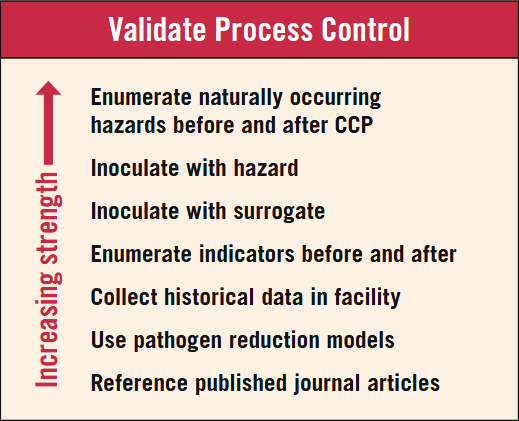
Guidance on conducting a robust validation
When preparing to conduct a validation study (Figure 1) to demonstrate effectiveness in pathogen control, it is important to consider the following:
Is the HACCP plan valid?
- Does it control the hazard?
- Can the plan be implemented as written?
When should I validate?
- Prior to implementing HACCP plan
- When changes in the process occur
- If a CCP, critical limits (CL), or monitoring method is changed
- If new food safety information becomes available
What should I validate?
- Primary chillers
- Post-chill immersion or spray bars
- Changed CL or CCPs
- Validity of the HACCP
- Lethality/stabilization (cook, chill)
The USDA-FSIS regulations require initial validations to be conducted prior to implementation of a HACCP plan and repeated testing of the adequacy of the CCPs, CL, monitoring, and record keeping procedures. It becomes even more critical to validate if your facility has frequent deviations from your HACCP plan or in the event of a recall (which indicates that your HACCP plan has failed). When setting up your in-plant validation studies, begin with relevant questions that can help you prove the safety of your product. For example, is your process safe? Can you change a CCP without compromising the safety of the product?
The next step in the process is to consult with your HACCP team and subject matter experts to determine where to find answers to your validation questions. Figure 1 provides a flow chart for determining the best validation option for your facility. Validations can be a tedious exercise, so assigning measurable variables such as temperature, time, pH, visual observations, and microbial data can streamline the process with fewer chances of error, if the parameters for the measurable variable are set accurately. A critical factor when designing your validation studies is the sampling plan, which makes your study statistically valid. Remember 100% assurance means testing 100% of the food produced, which is not practical and will leave no food for consumption. There is no way to completely avoid the risk of contaminated products when deciding how many samples to collect, however, a robust sampling plan allows you to select samples from the whole population to provide an adequate level of confidence that this subset would likely represent the whole population. You can reduce the risk by taking more samples, but you must seek a compromise between too large a sample with limited risk and a small sample with tremendous risk. The International Commission for Microbiological Specifications for Foods (ICMSF) categorizes hazards as moderate, serious, or severe, as follows:
| Moderate hazard | Serious hazard | Severe hazard |
|---|---|---|
| Illness is not usually life-threatening and is normally short in duration with self-limiting symptoms. | Illness is incapacitating but not usually life-threatening. | Illness is life-threatening for the general population or for restricted populations. |
If conducting a microbial validation study, consult a trained food microbiologist with knowledge of the product and the process for design, execution, analysis, and interpretation of the data.
Successful tips for validation studies
First, determine whether you need to conduct a validation study.
Then select a product or process.
- For example, whole carcass, cut-up parts, or further processed products
Select the relevant hazard for the product.
- In poultry, this means either Salmonella or Campylobacter.
- If indicators are to be used for routine validations, establish the correlation with the target pathogen.
- Decide whether to use single or multiple strains/serotypes. For multiple strains, at least five for each microorganism is recommended.
- Select strains/serotypes that are relevant and isolated from similar products
Decide what is relevant.
- For example, lab scale, pilot plant scale, and in-plant validation
Consider when and what to sample.
- Raw vs. finished product, before or after CCP, etc.
- Rinse sample, neck skin, sponge/swab sample, package rinse, etc.
Consider neutralization of the antimicrobial in the buffer.
- Neutralization can be conducted while collecting the sample in case of chemical interventions, if using antimicrobial interventions that result in residual antimicrobial on the sample.
Consider using methods to aid in the recovery of injured cells.
- Particularly when applying heat-treated and other physical antimicrobial treatments of products when conducting enumeration procedures (plating method)
Avoid bias.
- Form multidisciplinary teams.
- Conduct literature searches and scrutinize published data.
- When appropriate, incorporate the effects of microbial resistance, adaptation, cellular stresses, prior history of cells, and hurdle effects, etc.










